When a drug first hits the market, the company behind it gets about 20 years of protection from the date they file the main patent. But that’s not the end of the story. Many blockbuster drugs stay off-limits to generics for another 5, 10, even 15 years-not because of the original patent, but because of secondary patents. These aren’t about the active ingredient. They’re about the packaging, the timing, the form, or even how it’s used. And they’re the reason you’re still paying $500 for a pill that could cost $50.
What Exactly Is a Secondary Patent?
A secondary patent doesn’t protect the chemical that makes a drug work. It protects something around it: how it’s made, how it’s taken, or even a new disease it can treat. Think of it like this: the primary patent is the engine. The secondary patents are the wheels, the paint job, the GPS, and the sound system. All of them make the car feel new-even if the engine is old. For example, AstraZeneca’s Nexium wasn’t a new drug. It was just one half of the molecule in Prilosec. Prilosec’s patent was about to expire, so AstraZeneca isolated the active enantiomer (the S-isomer) and patented that version as Nexium. The drug worked the same way-but now, they had a new patent. That one move pushed generic competition back by nearly a decade. There are at least 12 types of secondary patents recognized globally. The most common ones include:- Formulation patents: Protecting how the drug is delivered-tablet, capsule, slow-release, or liquid. These make up about 22% of all secondary patents.
- Polymorph patents: Covering different crystal structures of the same molecule. GlaxoSmithKline used this to protect Paxil’s Form G, blocking generics for four extra years after the main patent expired.
- Method-of-use patents: Claiming a new medical condition the drug can treat. Thalidomide, once banned for causing birth defects, got a new patent for treating leprosy-and later, multiple myeloma. Each new use reset the clock.
- Composition patents: Protecting combinations of drugs in one pill. This is common for HIV and heart medications.
- Prodrug and metabolite patents: Covering how the body breaks down the drug into something active.
Why Do Companies Use Them?
It’s simple: money. The top-selling drugs make most of their revenue after the primary patent expires. But with secondary patents, companies can delay generics and keep prices high. A 2019 Health Affairs study found that drugs with secondary patents face generic competition 2.3 years later than those without them. Pfizer’s Lipitor, Merck’s Singulair, and AbbVie’s Humira are textbook cases. Humira, a biologic for autoimmune diseases, had over 260 secondary patents. The original patent expired in 2016. But because of the thick web of follow-up patents, generics didn’t arrive until 2023. During that gap, Humira brought in $20 billion a year. Without those patents, that number could’ve dropped by 80%. The business logic is ruthless but effective. Firms invest $12-15 million per secondary patent application. They hire teams of patent lawyers and scientists to file them years before the main patent runs out. The goal? Build a “patent thicket”-a maze of overlapping protections so complex that generic makers can’t afford to challenge them all.The Cost to Patients and Healthcare Systems
Patients don’t see patents. They see bills. And those bills are higher because of secondary patents. Pharmacy benefit managers like Express Scripts report that secondary patents raise their annual drug costs by 8.3%. That’s not a small number. It translates to billions more paid by insurers-and eventually, by you through higher premiums and out-of-pocket costs. Doctors notice it too. Many report “product hopping”-a tactic where companies push a new version of a drug right before generics hit. A patient on a cheap generic might be switched to a pricier branded version because the old one is no longer protected. The new version? Often no better. Just newer. Patient groups like Knowledge Ecology International point to Humira as proof. The drug cost $20 billion a year in the U.S. alone. With generics, it could’ve cost $4 billion. That’s $16 billion wasted on legal battles and marketing-not science. But it’s not all bad. Some secondary patents do bring real benefits. A 2021 American Cancer Society report found that new formulations of chemotherapy drugs reduced severe side effects by 37%. That’s meaningful. When a pill is easier to swallow, taken once a day instead of four, or doesn’t cause nausea, it improves lives. The problem isn’t innovation. It’s when innovation is used as a shield.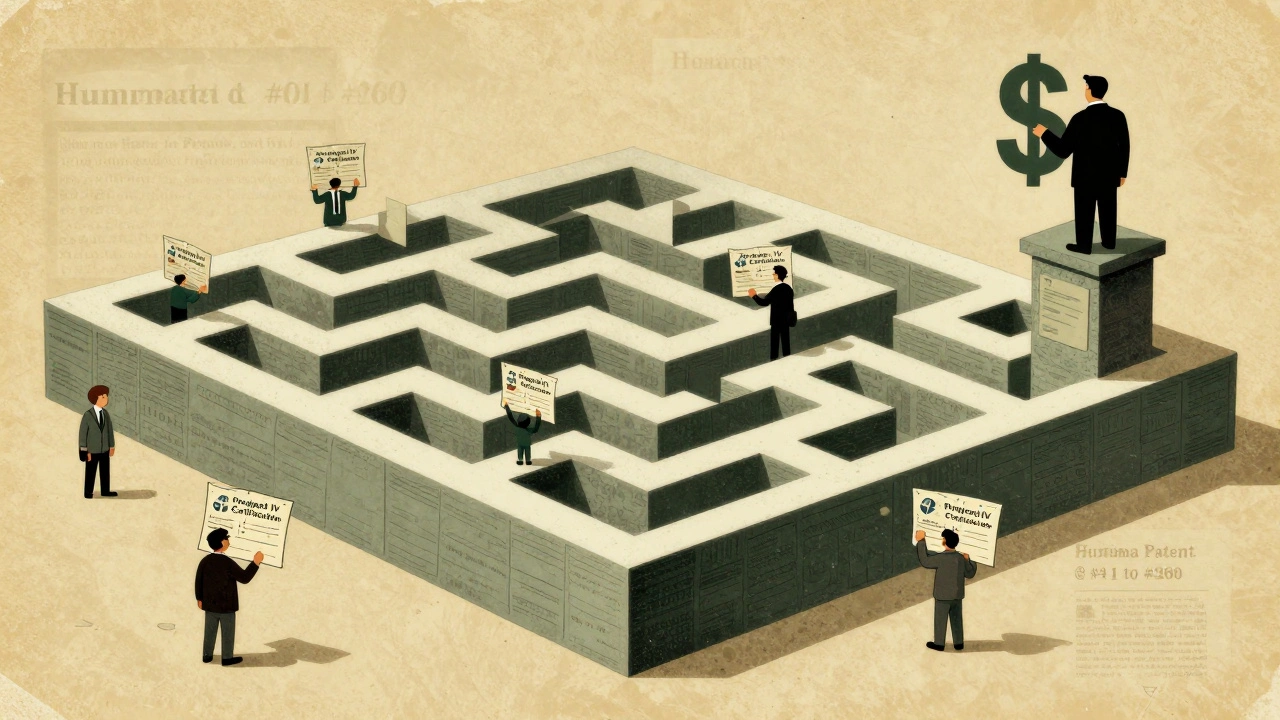
Global Differences: Who Allows This?
Not every country lets pharmaceutical companies play this game. India’s patent law, specifically Section 3(d) of the Patents Act (2005), says you can’t patent a new form of an old drug unless it shows “enhanced efficacy.” That’s why Novartis lost its fight to patent a new crystalline form of Gleevec in 2013. The court said: “It’s the same drug. Just a different shape.” Brazil requires approval from its health ministry before a patent can be enforced. That’s another barrier. In both countries, generics arrived years earlier than in the U.S. or Europe. The European Union is starting to crack down too. Their 2023 Pharmaceutical Strategy calls out “patent thickets” as a barrier to affordable medicine. The U.S. isn’t far behind. The 2022 Inflation Reduction Act lets Medicare challenge certain secondary patents-something it couldn’t do before. The World Health Organization now lists secondary patents as the top legal reason why essential medicines stay expensive in 68 low- and middle-income countries.How Generic Companies Fight Back
Generic manufacturers don’t just sit back. They fight. In the U.S., they file “Paragraph IV certifications” when they believe a secondary patent is invalid or won’t be enforced. In 2022, 92% of listed secondary patents were challenged this way. But only 38% of those challenges succeeded in court. Why so low? Because even if a patent is weak, the legal costs are huge. It takes $15-20 million and 3.2 extra years on average to get a generic approved when secondary patents are involved. Some companies are now using “patent watch” teams that track potential lawsuits 7-10 years in advance. They don’t just wait for a patent to expire. They build legal strategies years ahead.








Write a comment
Your email address will be restricted to us